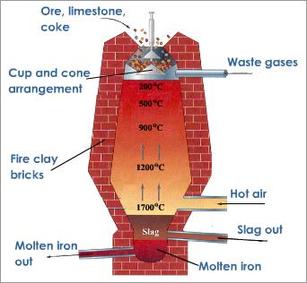
Chemical Reactions of Lead Smelting
A major and primary Lead mineral is galena (chemical formula = PbS) which comprises of 86.6% of Lead [1]. In order to smelt this mineral, a blast furnace is needed to be used. A blast furnace is an enormous oven which is used to accomplish the smelting processes [2]. The process in which galena is smelted requires two important chemical reactions to occur within the furnace.
1) The galena is roasted (reacts with O2) in order to remove the sulfur component of the metal sulfide [2]. Roasting is a method where a sulfide ore (i.e an ore containing PbS) is heated in air which converts the metal sulfide to either a metal oxide or a metal itself [3].
Word Equation: Lead (II) Sulfide + Oxygen à Lead (II) Oxide + Sulfur Dioxide
Chemical Equation: 2PbS + 3O2 à 2PbO + 2SO2
2) The newly formed Lead (II) Oxide subsequently reacts with coke to attain refined Lead [2]. Coke is a pure form of coal that contains carbon and is essential in the extraction of metals from their oxides [2].
Word Equation: Lead (II) Oxide + Carbon à Lead + Carbon Dioxide
Chemical Equation: 2PbO + C à 2Pb + CO2
There is another process in which the galena mineral can be smelted through [4]. It also requires two chemical reactions to occur within the furnace.
1) Due to the sulfur content, carbon from the coke will not be able to reduce lead. Therefore, the mineral must be roasted to oxidize sulfur and create a metal oxide out of galena.
Word Equation: Lead (II) Sulfide + Oxygen à Lead (II) Oxide + Sulfur Dioxide
Chemical Equation: 2PbS + 3O2 à 2PbO + 2SO2
2) Raw ore is then added to the products and is heated further.
Word Equation: Lead (II) Oxide + Lead (II) Sulfide à Lead + Sulfur Dioxide
Chemical Equation: 2PbO + PbS à 3Pb + SO2
REFERENCES:
[1] http://www.buzzle.com/articles/uses-of-lead.html
[2] http://qldscienceteachers.tripod.com/junior/chem/metals.html
[3] http://chemistry.about.com/od/chemistryglossary/a/roastingdef.htm
[4] http://mysite.du.edu/~jcalvert/phys/lead.htm#Prod